Phosphine-catalyzed remote α-C–H bond activation of alcohols or amines triggered by the radical trifluoromethylation of alkenes: reaction development and mechanistic insights†
Abstract
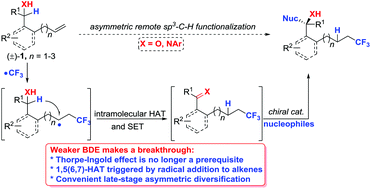
Intramolecular hydrogen atom transfer (HAT) for the remote functionalization of C(sp3)–H bonds has emerged as a powerful strategy, but its asymmetric diversification remains a great challenge because of the requirement of harsh reaction conditions and less enantiotopic discrimination. To overcome this, we described a general and efficient radical protocol for the concomitant functionalization of both alkenes and the remote α-C–H bonds of alcohols or amines via 1,5(6,7)-HAT triggered by the addition of a trifluoromethyl radical to alkenes in a highly controlled site-selective manner. Furthermore, such an approach could be developed for late-stage asymmetric diversification at the remote sp3-hydridized positions of alcohols or amines via a cascade sequence for the facile construction of chiral CF3-containing homoallylic alcohols or secondary amines with good to excellent enantioselectivities. Mechanistic experiments and DFT calculations revealed that 1,5(6,7)-HAT is a kinetically relevant process and provided a rationale for the observed different reactivities between the linear alkenyl alcohol or amine and alkenyl ketone or amide.


 Please wait while we load your content...
Please wait while we load your content...