An unexpected acid-catalyzed decomposition reaction of cilnidipine and pranidipine to the decarboxylative bridged tricyclic products via cascade rearrangements†
Abstract
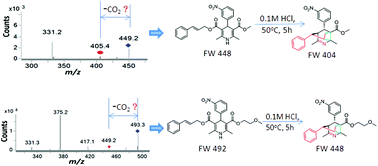
A gas-phase Carroll rearrangement occurring during electrospray ionization tandem mass spectrometry (ESI-MS/MS) led to the discovery of bridged tricyclic degradation products from cilnidipine and pranidipine under acidic conditions for the first time. The unexpected acid-catalyzed decomposition product of cilnidipine was separated and identified by MS, NMR and X-ray analyses. An acid-catalyzed Carroll rearrangement, a hetero-Diels–Alder reaction and a sigmatropic rearrangement cascade reaction were proposed for the formation of bridged tricyclic degradation products from cilnidipine and pranidipine under acidic conditions. Theoretical calculations supported the proposed reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...