Multifunctional odorless isocyano(triphenylphosphoranylidene)-acetates: synthesis and direct one-pot four-component Ugi/Wittig cyclization to multisubstituted oxazoles†
Abstract
The multifunctional odorless isocyano(triphenylphosphorany-lidene)acetate was prepared as a new stable phosphorus ylide containing isocyanide group. A new one-pot four component synthesis of multisubstituted oxazoles by a cascade Ugi/Wittig process without Mumm rearrangement has been developed, starting from the isocyano(triphenylphosphora-nylidene)acetates, aldehydes, amines and acids. Two component reactions of isocyano(triphenylphosphoranylidene)-acetates and acids also generated disubstituted oxazoles in good yields.
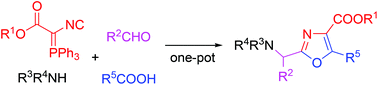


 Please wait while we load your content...
Please wait while we load your content...