Garciyunnanimines A–C, novel cytotoxic polycyclic polyprenylated acylphloroglucinol imines from Garcinia yunnanensis†
Abstract
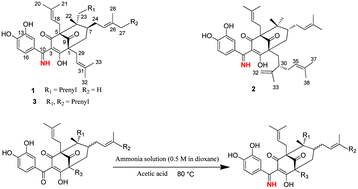
Three novel polycyclic polyprenylated acylphloroglucinol imines (PPAP imines), garciyunnanimines A–C (1–3), which contain unprecedented imine functionality at C-10, together with their presumed biogenetic precursors, guttiferone K (6), garcinol (7) and oblongifolin C (8), were isolated from Garcinia yunnanensis. The imines’ synthesis pathways and structures were elucidated by conversion of the known PPAPs 6–8 to the corresponding imines 1–3 in good yields in the presence of ammonia in dioxane and acetic acid. Using the established method, two new PPAP imine derivatives, garciyunnanimines D and E (4 and 5), were easily synthesized using the corresponding PPAPs, oblongifolins A and B (9 and 10). Structures of the new compounds were assigned by spectroscopic analysis and verified by biomimetic conversion and calculations of the ECD (electron circular dichroism) spectra. In addition, the cytotoxicities of these compounds were evaluated using a CCK-8 assay against three human cancer cell lines, A549, HepG2, and RPMI-8226.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...