Visible-light photoredox catalyzed hydroacylation of electron-deficient alkenes: carboxylic anhydride as an acyl radical source†
Abstract
A convenient method mediated by photoredox catalysis for the direct construction of 1,4-dicarbonyl compounds has been developed. Simple aromatic symmetric carboxylic anhydrides or mixed anhydrides (generated in situ) have been utilized as an acyl radical source in addition to different types of Michael acceptors. A wide range of substrates are amenable to this new type of acyl Michael addition under mild conditions with broad functional group tolerance. As an application, a novel four-step synthesis of medicinal agent haloperidol is also presented.
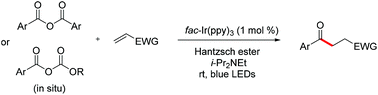


 Please wait while we load your content...
Please wait while we load your content...