meta-Selective C–H difluoromethylation of various arenes with a versatile ruthenium catalyst†
Abstract
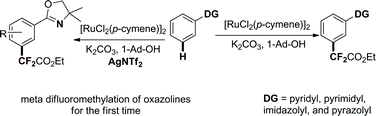
A ruthenium-enabled meta-selective C–H difluoromethylation of arenes has been developed. Various arenes bearing pyridyl, pyrazolyl, imidazolyl, or pyrimidyl directing groups, or removable oxazoline directing groups, were tolerated in this meta-selective C–H difluoromethylation, affording the difluoromethylated products in moderate to good yields. The difluoromethyl group could be selectively installed into several drug molecules and bioactive compounds in one step, highlighting the synthetic utility and importance of this new method.


 Please wait while we load your content...
Please wait while we load your content...