Selective cross-dehydrogenative C–O coupling of N-hydroxy compounds with pyrazolones. Introduction of the diacetyliminoxyl radical into the practice of organic synthesis†
Abstract
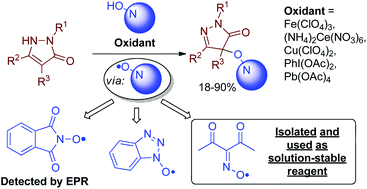
Oxidative C–O coupling of pyrazolones with N-hydroxy compounds of different classes (N-hydroxyphthalimide, N-hydroxybenzotriazole, oximes) was achieved; both one-electron oxidants (Fe(ClO4)3, (NH4)2Ce(NO3)6) and two-electron oxidants (PhI(OAc)2, Pb(OAc)4) are applicable, and the yields reach 91%. Apparently, the coupling proceeds via the formation of N-oxyl radicals from N-hydroxy compounds. One of the N-oxyl intermediates, the diacetyliminoxyl radical, was found to be exclusively stable in solution in spite of being sterically unhindered; it was isolated from an oxidant and used as a new reagent for the synthesis and mechanism study. The products of C–O coupling of pyrazolones with N-hydroxyphthalimide can be easily transformed into aminooxy compounds, valuable substances for combinatorial chemistry.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...