Post-synthetic diversification of pyrrole-fused benzosultams via trans-sulfonylations and reactions on the periphery of pyrrole†
Abstract
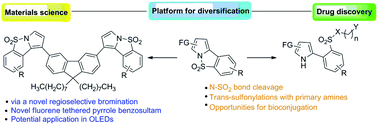
The chemistry (synthesis and reactions) of pyrrole-fused benzosultams has been the subject of this current investigation. Their workable access via palladium-catalyzed oxidative coupling has made this study feasible. The trans-sulfonylation in pyrrole-fused benzosultams provided access to otherwise unattainable (NH)-2-arylpyrroles containing an ortho-sulfonamide functionality. Other N–SO2 bond cleavages in pyrrole-fused benzosultams yielded synthetically useful (NH)-2-arylpyrroles containing other ortho-sulfonyl functionality. A terminal amine or hydroxyl functionality, ideally poised for bioconjugation, is another important feature of some of the synthesized (NH)-2-arylpyrroles. The rationally designed and synthesised novel fluorenes tethered with pyrrole-fused benzosultam could find applications as organic emitters in organic light-emitting devices (OLEDs).


 Please wait while we load your content...
Please wait while we load your content...