Computational study on NHC-catalyzed enantioselective and chemoselective fluorination of aliphatic aldehydes†
Abstract
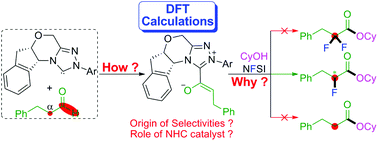
In this work, the reaction mechanism, stereoselectivity, and chemoselectivity of oxidative α-fluorination of aliphatic aldehydes enabled by N-heterocyclic carbene catalysts have been investigated using a density functional theory (DFT) method. The computed outcomes reveal that the whole reaction includes several processes, i.e., formation of the Breslow intermediate, oxidative deprotonation to give the cation intermediate, deprotonation of the α-C(sp3)–H bond, α-fluorination, esterification by CyOH affording the α-fluoroester, and dissociation of the catalyst and the final product. According to the computational results, the fluorination process was identified to be the stereoselectivity-determining step. The addition on the different prochiral faces of the enolate intermediate results in the formation of a stereoselective fluorinated intermediate and the chiral center assigned to the α-carbon emerges during the fluorination process. Non-covalent interaction (NCI) analysis revealed the presence of relatively stronger non-covalent interactions (i.e. π–π stacking), which leads to the predominant position of the S-configurational reaction pathway. Moreover, the competing reaction pathways affording the by-products of aliphatic ester and difluorinated ester were calculated to be kinetically unfavorable. Furthermore, global reactivity index (GRI) analysis shows that NHC promotes the reaction by enhancing the nucleophilicity of the enolate intermediate and the electrophilicity of the monofluorinated intermediate. The obtained theoretical insights would be useful for rational design of the fluorination of aliphatic aldehydes with high selectivity under organocatalysis.


 Please wait while we load your content...
Please wait while we load your content...