Chromium-catalyzed migratory arylmagnesiation of unactivated alkynes†
Abstract
We report here on a chromium-catalyzed addition reaction of an arylmagnesium bromide to an unactivated internal alkyne to afford an ortho-alkenylarylmagnesium bromide. This reaction likely proceeds via the insertion of an alkyne into an arylchromium species, alkenyl-to-aryl 1,4-chromium migration, and transmetalation between the resulting arylchromium species and the starting aryl Grignard reagent. The product of this reaction is amenable to a series of electrophilic trapping reactions.
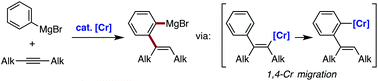


 Please wait while we load your content...
Please wait while we load your content...