Platinum-catalyzed syn-stereocontrolled ring-opening of oxabicyclic alkenes with sodium arylsulfinates†
Abstract
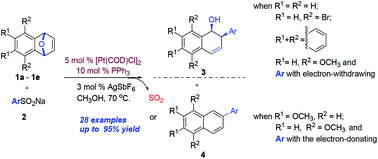
A new efficient platinum-catalyzed ring-opening reaction of oxabicyclic alkenes with a wide range of sodium arylsulfinates was developed, affording the desired products 3 or 4 in good to excellent yields under mild conditions. This protocol provides a new low-cost, viable and convenient method toward the synthesis of cis-2-aryl-1,2-dihydronaphthalen-1-ols 3 and 2-aryl-naphthalenes 4 with good functional group tolerance. To the best of our knowledge, it represents the first example in the ring-opening reactions of oxabicyclic alkenes with sodium arylsulfinates via a platinum catalyst and desulfonylation using the Pt catalyst. In addition, a plausible mechanism for the ring-opening reaction was also proposed.

 Please wait while we load your content...
Please wait while we load your content...