Efficient trifluoromethylation via the cyclopropanation of allenes and subsequent C–C bond cleavage†
Abstract
As we know, the incorporation of a trifluoromethyl group into organic molecules may significantly alter their physical and biological properties due to the high electronegativity, lipophilicity, and excellent metabolic stability of the trifluoromethyl substituent. Thus, an efficient method for the introduction of the trifluoromethyl group is of high current interest. On the other hand, vinylic cyclopropanes are a class of strained compounds capable of undergoing ring-opening reaction with other molecules. Here, CF3-substituted vinylic cyclopropanes have been highly selectively formed by a copper-catalyzed cyclic trifluoromethylation of (4,4-disubstituted-2,3-butadienyl)malonates with Togni's reagent II, in which the trifluoromethyl group was installed at the middle carbon of the allene unit by applying 1,10-phenanthroline as the ligand. Such unique cyclopropanes successfully bring the trifluoromethyl group to other useful organic skeletons by the selective cleavage of C–C bonds with an exclusive diastereoselectivity. Based on the mechanistic studies, an allene radical addition, oxidation, and allylic substitution pathway has been proposed.
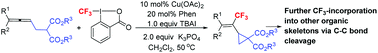
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...