TBAF catalyzed one-pot synthesis of allenyl-indoles†
Abstract
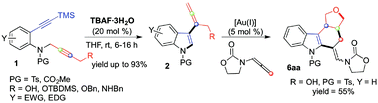
The site selective synthesis of functionalized indoles is presented under environmentally convenient tetrabutylammonium fluoride catalysis. The metal-free approach exploits the combined efficiency of Bu4N+ and F− ions in performing a cascade sequence involving intramolecular hydroamination of the C–C triple bond, cleavage of silyl-protecting groups and site-selective sigmatropic aza-Cope-type [3,3]-rearrangement. A range of C(3)-allenyl-(1H)-indoles (2) is obtained in an efficient manner (yield up to 93%) providing a valuable synthetic alternative to noble metal-based protocols.


 Please wait while we load your content...
Please wait while we load your content...