Iridium-catalyzed direct asymmetric reductive amination of aromatic ketones†
Abstract
The highly efficient direct catalytic reductive amination of aromatic ketones with diphenylmethanamine is described. Catalyzed by the iridium–monodentate phosphoramidite complex and assisted using additives, this concise and practical route provided chiral amines in high yields and with high enantioselectivity (ee values of up to 99%).
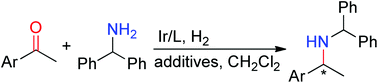
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...