An improved methodology for the synthesis of 1α,25-dihydroxy-20-epi-vitamin D3 (MC 1288) and Gemini analog Ro-438-3582†
Abstract
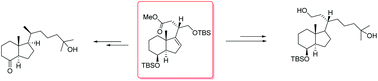
Control over the C-20 stereochemistry allows a versatile method to introduce novel side-chains into the vitamin D scaffold. Using our building blocks containing stereodefined stereochemistry at C-20 we demonstrated that we could improve the current synthesis of calcitriol analogs with potentially interesting biological properties.


 Please wait while we load your content...
Please wait while we load your content...