Nickel-catalyzed highly chemo- and stereoselective borylative cyclization of 1,6-enynes with bis(pinacolato)diboron†
Abstract
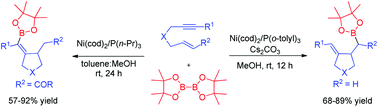
A highly chemo- and stereoselective nickel-catalyzed borylative cyclization of 1,6-enynes with bis(pinacolato)diboron is described. Enynes containing an electron-deficient alkene such as a vinyl ketone provided alkenylboronates while enynes containing a terminal alkene gave homoallylic boronates. A plausible mechanism involving a nickelacyclopentene intermediate is proposed. In addition, the resulted alkenyl- or homoallylic boronates can be transformed to the corresponding ketones and alcohols via oxidation. Application to a palladium-catalyzed cross coupling reaction of alkenylboronates with aromatic halides has been demonstrated as well.


 Please wait while we load your content...
Please wait while we load your content...