Metal-free phosphonation of benzoxazoles and benzothiazoles under oxidative conditions†
Abstract
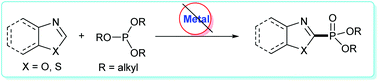
An iodine promoted phosphonation of benzoxazoles and benzothiazoles with trialkyl phosphites was described. This reaction undergoes three steps: an addition procedure, followed by a radical oxidation, and an elimination reaction, provides an efficient method to access 2-phosphated products with a broad scope of substrates.


 Please wait while we load your content...
Please wait while we load your content...