Synthesis of 2,3-dihydro-1H-phosphindole-1-oxides via the t-BuLi-mediated rearrangement of vinyl bromides and phosphine oxides†
Abstract
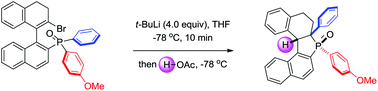
We report an intramolecular addition reaction of vinyllithium to phosphine oxides for the efficient synthesis of 2,3-dihydro-1H-phosphindole-1-oxides. Control experiments indicated that the reaction might proceed through an addition and concerted aryl migration process. The relative migration rate of aryl rings with different electronic properties was also briefly investigated.


 Please wait while we load your content...
Please wait while we load your content...