Merging photoredox catalysis with transition metal catalysis: site-selective C4 or C5-H phosphonation of 8-aminoquinoline amides†
Abstract
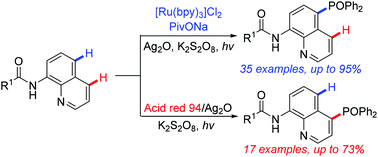
A simple and efficient protocol for the transition metal-catalyzed site-selective C–H phosphonation of 8-aminoquinolines with diarylphosphine oxide or H-phosphonate diesters was developed via a photoredox process. This reaction features high regioselectivity at the C4 or C5 position under mild and simple conditions (with a catalytic amount of silver salt at room temperature). Note that the C4 position of 8-aminoquinoline is an unusual reactive site.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...