Thiophene–pyrrole containing S,N-heteroheptacenes: synthesis, and optical and electrochemical characterisation†
Abstract
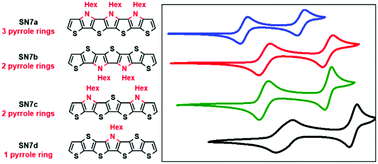
A novel family of S,N-heteroheptacenes SN7a–d with a variable thiophene–pyrrole ratio and a heteroring fusion sequence is presented. All SN7-derivatives can be synthesized in efficient multi-step synthetic routes with good overall yields. The crucial cyclization step to the stable and soluble fused systems is achieved by multiple Pd-catalyzed Buchwald–Hartwig aminations or C–S coupling reaction in high yields. The comparison of the optoelectronic properties provides interesting structure–property relationships and gives valuable insights into the role of nitrogen atoms within the series of thiophene–pyrrole containing S,N-heteroheptacenes. Remarkable differences in emission behaviour and noticeable correlations of the oxidation potentials of the S,N-heteroheptacenes offer helpful information for the construction of other heteroacenes. Additionally, UV-Vis-NIR absorption spectra of the corresponding radical cations generated by chemical oxidation were monitored and compared.


 Please wait while we load your content...
Please wait while we load your content...