A photoinduced reaction of perfluoroalkyl halides with 1,3-diarylprop-2-yn-1-ones catalyzed by DABSO†
Abstract
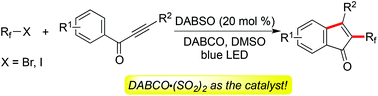
The sulfur dioxide surrogate of DABCO·(SO2)2 is used as an efficient catalyst for the cleavage of the perfluoroalkyl-halogen bond under visible-light irradiation for the first time. This unexpected reaction of perfluoroalkyl halides and 1,3-diarylprop-2-yn-1-ones catalyzed by DABCO·(SO2)2 in the presence of visible light proceeds smoothly, leading to 3-fluorinated indenones in good yields. During the reaction process, the cleavage of the perfluoroalkyl-halogen bond and subsequent radical oxidative cyclization are involved under metal-free conditions. A broad reaction scope is observed. Moreover, a plausible mechanism supported by theoretical calculations is proposed.


 Please wait while we load your content...
Please wait while we load your content...