Pentadecaphenylenes: synthesis, self-assembly and complexation with fullerene C60†
Abstract
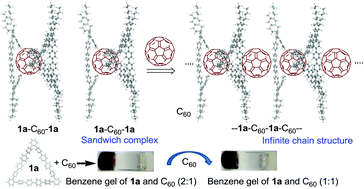
Triangular macrocyclic pentadecaphenylenes have been synthesized in moderate yields by the electron-transfer oxidation of the corresponding Lipshutz cuprates with duroquinone. Pentadecaphenylenes are very stable to light, atmospheric oxygen and prolonged heating. Despite the rigid twisted frame, pentadecaphenylenes self-assemble into various nanostructured polymorphs in the solid state. Hexaoctylpentadecaphenylene 1a acts as a gelator in benzene or toluene to form a gel, although 1a neither has a planar structure nor undergoes heteroatom substitution to assist intermolecular interaction. Furthermore, 1a incorporated fullerene C60 into its cavity to afford a fibrous 2 : 1 sandwich complex (1a–C60–1a) which showed higher gelation ability than 1a itself. Interestingly, Janus-faced 1a acts as a host of two C60 molecules, and an additional C60 was intercalated into the 1a·C60 (2 : 1) complex to form a 1 : 1 complex with an infinite chain structure like –1a–C60–1a–C60–, which enhances the gelation power of the 1a·C60 sandwich complex.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...