Chemoenzymatic preparation of optically active cyclic 4-hydroxy-acylaziridines†
Abstract
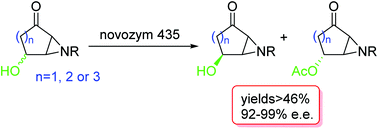
(x + 1)-Hydroxyazabicyclo[x.1.0]alkane-2-ones are useful intermediates for organic synthesis. A method for the preparation of these bicyclic 4-hydroxy-acylaziridines in optically active form is described. Stereoselective aziridination of racemic protected 4-hydroxycycloenones followed by enzymatic resolution with Novozym 435 (CAL-B) afforded the chiral hydroxyaziridines in good yields and enantiomeric excess (up to 99%). The described method is simple, reproducible and scalable. A stereoselective tandem elimination followed by 1,4-addition on a cyclopentanone is also described.

 Please wait while we load your content...
Please wait while we load your content...