An expedient E-stereoselective synthesis of multi-substituted functionalized allylic boronates from Morita–Baylis–Hillman alcohols†
Abstract
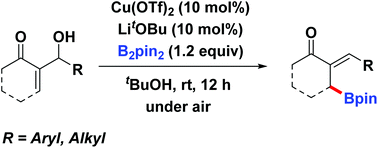
An expedient and stereoselective synthesis of functionalized trisubstituted allylic boronates via direct borylation of Morita–Baylis–Hillman alcohols is disclosed. The reaction proceeds smoothly at ambient temperature with high atomic and synthetic efficiency. The desired allylic boronates can be applied in the efficient synthesis of homoallyl alcohols and α-exomethylene γ-lactones.


 Please wait while we load your content...
Please wait while we load your content...