Pincer cobalt complex-catalyzed Z-selective hydrosilylation of terminal alkynes†
Abstract
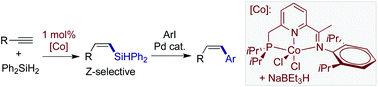
A phosphine-iminopyridine (PCNN) cobalt-catalyzed Z-selective hydrosilylation of terminal alkynes with Ph2SiH2 has been developed for the synthesis of (Z)-β-vinylsilanes with high regio- and stereoselectivity and wide functional group tolerance. Furthermore, the Co-catalyzed hydrosilylations of unsymmetrical arylalkyl disubstituted internal alkynes afford syn-addition products with unique regioselectivity: the silyl group is added to the alkyl-substituted carbon, instead of the aryl-substituted carbon. The (Z)-β-vinylsilane products are further applied to Pd-catalyzed Hiyama–Denmark cross-couplings for stereoselective synthesis of (Z)-disubstituted alkenes.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry

 Please wait while we load your content...
Please wait while we load your content...