A new approach to arylhydrazides via the reaction of the Mitsunobu reagent with arynes: further application to access diverse nitrogen-containing heterocycles in one pot†
Abstract
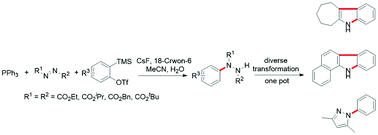
A new route to arylhydrazides involving the reaction of two highly active intermediates, the 1,3-zwitterion generated in situ from the Mitsunobu reagent and arynes, under mild conditions has been developed. Further transformations of the versatile arylhydrazides to diverse nitrogen-containing heterocycles in one pot are also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...