Diastereoselective synthesis of fluoroisosteric analogues of antiparasitic pyrrolobenzoxazine alkaloids from tryptophan by successive fluorination–cyclization and a Meisenheimer-type rearrangement†
Abstract
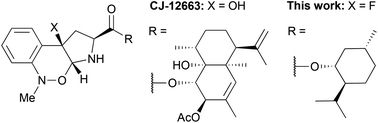
Fluoroisosteric analogues of an antiparasitic pyrrolobenzoxazine alkaloid CJ-12663 were designed and diastereoselectively synthesized by successive fluorination–cyclization and a Meisenheimer-type rearrangement from tryptophan in good yields.


 Please wait while we load your content...
Please wait while we load your content...