Introducing topology to assess the synchronicity of organic reactions. Dual reactivity of oximes with alkenes as a case study†‡
Abstract
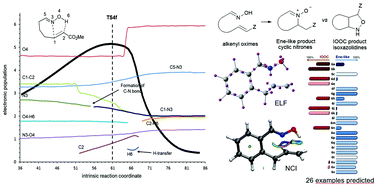
The synchronicity of organic reactions not always can be determined by only the analysis of transition structures. We present a case study that illustrates that topological analyses provide information regarding synchronicity that, often, is not reflected in the geometry of transition structures. We have chosen the competitive reactions of oximes with alkenes, (3 + 2) dipolar cycloaddition and ene-like reaction, which have been computationally studied to determine the parameters favoring each process. The competition between the two reactions is particularly evidenced in alkenyl oximes leading to intramolecular processes. Up to 26 examples of intramolecular reactions have been calculated and the results predicted the favored process.


 Please wait while we load your content...
Please wait while we load your content...