DMSO-promoted regioselective synthesis of sulfenylated pyrazoles via a radical pathway†
Abstract
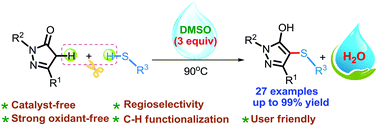
A facile and eco-friendly protocol for the construction of C-4 sulfenylated pyrazoles via a radical pathway was established for the first time. The reaction worked smoothly under catalyst- and solvent-free conditions to afford a wide range of sulfenylated pyrazole derivatives in good to excellent yields. The thiyl free radical generated in situ, here, also served as a single electron transfer medium for the present transformation. This reaction provides a new strategy for the formation of C–S bonds.


 Please wait while we load your content...
Please wait while we load your content...