Intramolecular aminocyanation of alkenes promoted by hypervalent iodine†
Abstract
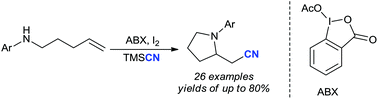
A transition metal-free and hypervalent iodine-promoted annulative aminocyanation of unactivated alkenes has been developed for the first time. This regioselective 5-exo-trig process provides a concise method to synthesize diversely substituted cyanated pyrrolidine, piperidine and indoline derivatives under mild reaction conditions with up to 80% yield, in which the cyanated position of the double bond is controlled by different hypervalent iodine regents.

 Please wait while we load your content...
Please wait while we load your content...