Nickel-catalyzed selective C-5 fluorination of 8-aminoquinolines with NFSI†
Abstract
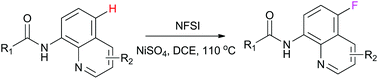
The first nickel-catalyzed C–H selective fluorination of 8-aminoquinoline derivatives at the C-5 position was achieved using NFSI (N-fluorobenzenesulfonimide) as the “F” source and NiSO4 as the catalyst. This method demonstrated broad substrate tolerance under mild conditions and a plausible SET (single electron transfer) mechanism was suggested in the proposal of a radical process.

 Please wait while we load your content...
Please wait while we load your content...