Concise catalytic asymmetric total syntheses of ancistrocladinium A and its atropdiastereomer†
Abstract
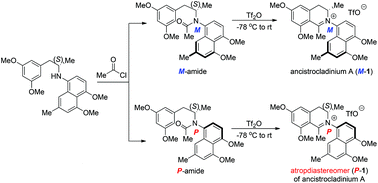
Asymmetric total syntheses of ancistrocladinium A and its atropdiastereomer, in an atropisomerically pure form, are described. Chiral phosphoric acid-catalyzed asymmetric reductive amination of 1-aryl propanone and naphthylamine followed by acetylation provided two atropisomerically pure tertiary amides. Subsequent stereospecific Bischler–Napieralski reaction of each of these tertiary amides with triflic anhydride provided ancistrocladinium A or its atropdiastereomer, as a single atropisomer, respectively.

 Please wait while we load your content...
Please wait while we load your content...