Synthesis of 2,3-diaza-anthraquinones via the bidentate Lewis acid catalysed inverse electron-demand Diels–Alder (IEDDA) reaction†
Abstract
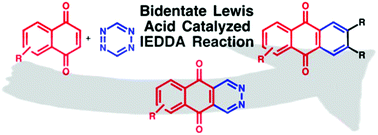
The bidentate bisborane Lewis acid catalysed inverse electron-demand Diels–Alder (IEDDA) reaction of 1,4-naphthoquinones (1,4-NQs) with 1,2,4,5-tetrazine (TZ) has been developed for the synthesis of 2,3-diaza-anthraquinones (2,3-DAAQs). Only due to the coordination of TZ with the bisborane catalyst, the IEDDA reaction of electron-deficient 1,4-NQs is possible. The use of TZ as the nitrogen source provides an efficient strategy to the 2,3-diaza-anthraquinones. The 2,3-DAAQs themselves are suitable dienes for a second bidentate Lewis acid catalysed IEDDA reaction with various dienophiles to afford substituted AQs. AQs have been discussed as privileged structures for applications in medicinal as well as materials science.
- This article is part of the themed collection: Novel π-electron molecular scaffolds

 Please wait while we load your content...
Please wait while we load your content...