Transition-metal-free oxidative reaction of hydrazines and potassium metabisulfite for preparation of sulfonohydrazides†
Abstract
A transition-metal-free oxidative coupling reaction for the synthesis of sulfonohydrazides from two types of hydrazines and potassium metabisulfite under air has been developed. This highly selective reaction used one arylhydrazine as an aryl coupling partner and potassium metabisulfite as a sulfur dioxide precursor, which provides a new and green strategy to sulfonohydrazides.
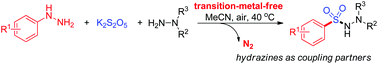


 Please wait while we load your content...
Please wait while we load your content...