Effect of Lewis acid bulkiness on the stereoselectivity of Diels–Alder reactions between acyclic dienes and α,β-enals†
Abstract
The factors controlling the reactivity and endo/exo selectivity of Lewis acid-catalysed Diels–Alder reactions between highly substituted open-chain 1,3-dienes and α,β-enals have been explored computationally by means of density functional theory calculations. In agreement with previous experimental observations, it is found that the B(C6F5)3-catalysed cycloaddition reaction leads almost exclusively to the corresponding exo-cycloadduct, whereas the analogous AlCl3-mediated process is highly endo-selective. The effect of Lewis acid bulkiness on stereoselectivity has been quantitatively analysed by means of a combination of the activation strain model of reactivity and the energy decomposition analysis methods. In contrast to the current view, the exo-selectivity promoted by the bulky B(C6F5)3 catalyst does not result from the steric destabilization of the corresponding endo-transition state but from the interplay between the less destabilizing strain energy and the stronger interaction between the deformed reactants along the entire reaction coordinate. In addition, non-covalent interactions are found to play a crucial role in stabilizing the exo-approach. These results allow us to not only quantitatively understand the effect of the Lewis acids in the process, but also predict new catalysts leading to highly exo-selective Diels–Alder reactions.
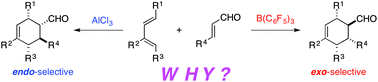


 Please wait while we load your content...
Please wait while we load your content...