Visible light-induced tandem oxidative cyclization of 2-alkynylanilines with disulfides (diselenides) to 3-sulfenyl- and 3-selenylindoles under transition metal-free and photocatalyst-free conditions†
Abstract
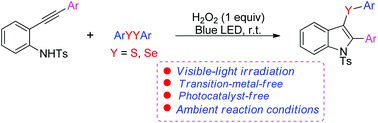
A simple and efficient strategy for the synthesis of 3-sulfenyl and 3-selenyl indoles via a visible light-induced tandem cyclization of 2-alkynylanilines with disulfides (diselenides) was developed. The reaction generated the corresponding products in good yields at room temperature under transition metal-free and photocatalyst-free conditions.


 Please wait while we load your content...
Please wait while we load your content...