Copper-catalyzed oxidative amidation of α,β-unsaturated ketones via selective C–H or C–C bond cleavage†
Abstract
The copper-catalyzed oxidative amidation of readily available α,β-unsaturated ketones with N-fluorobenzenesulfonimide (NFSI) for the synthesis of biologically active enamides or α-amino substituted unsaturated ketone skeletons has been achieved. These transformations processed via a radical pathway, thus the amidation occurred at the unusual α-position of the α,β-unsaturated ketones, leading to the following effective C(CO)–C(vinyl) or C(vinyl)–H bond cleavage with high selectivity.
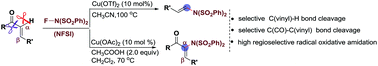
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...