Enantioselective synthesis of a cyclobutane analogue of Milnacipran†
Abstract
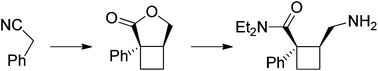
The asymmetric synthesis of a cyclobutane analogue of the antidepressant drug Milnacipran is reported. The optically active derivative incorporates a central cyclobutane ring in lieu of the cyclopropane unit classically found in Milnacipran. The two stereogenic centres borne by the cyclobutane were sequentially installed starting from phenylacetonitrile.


 Please wait while we load your content...
Please wait while we load your content...