Ruthenium(ii)-catalyzed C–H alkenylation/annulation cascade for the rapid synthesis of benzoimidazoisoindoles†
Abstract
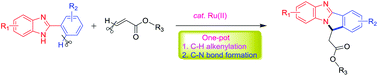
An efficient one pot synthesis of benzimidazole-fused isoindoles from 2-arylbenzimidazole and readily available conjugated alkenes has been explored. The developed domino strategy involved the ruthenium-catalyzed formation of two distinct C–C and C–N bonds and one new five-membered ring to afford a polycyclic molecule. The rapid and base-free reaction conditions and the broader substrate scope are salient features of this novel protocol. The ruthenium-catalyzed tandem reaction provides a direct tool for the easy and rapid access to a new benzoimidazoisoindole scaffold.

 Please wait while we load your content...
Please wait while we load your content...