Electronic and steric effects on the three-fold Scholl-type cycloheptatriene ring formation around a tribenzotriquinacene core†‡
Abstract
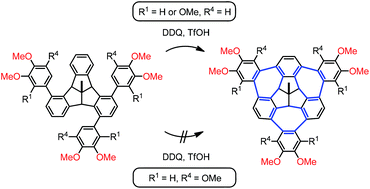
Systematic studies on the role of substituents in the bay-bridging cycloheptatriene ring formation around the tribenzotriquinacene (TBTQ) core via the non-typical Scholl reaction were carried out. The electronic effect of the substituents was found to be the predominant factor that controls the ease of the cyclization reaction, while the steric effect of methoxy groups in the bay regions of the TBTQ core appears to be also significant but less important. In several cases with insufficient electronic activation and/or unfavorable steric restriction, single bay-bridging occurred with or without concomitant bridgehead hydroxylation. Alternatively, an unprecedented ring opening/closure of the TBTQ skeleton by electrophilic ipso-attack was found to intervene in other cases. Starting from the electronically and sterically most favorable precursor, a 1,4,8-tris-(2,3,4-trimethoxyphenyl)-TBTQ derivative, a new wizard-hat-shaped, three-fold bay-bridged TBTQ nanographene core bearing nine methoxy groups at the molecular periphery was synthesized with high efficiency.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...