Asymmetric amination of 2-substituted indolin-3-ones catalyzed by natural cinchona alkaloids†
Abstract
The natural quinine-catalyzed enantioselective amination of 2-substituted indolin-3-ones with azodicarboxylates has been developed. These reactions provide a broad spectrum of 2,2-disubstituted indolin-3-ones containing a nitrogen-attached quaternary stereocenter at the C2-position in extremely high yields and with excellent enantioselectivities. The readily available catalyst, broad substrate scope, and mild reaction conditions highlight the practical utility of this process.
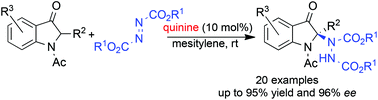


 Please wait while we load your content...
Please wait while we load your content...