Generation of benzosultams via a radical process with the insertion of sulfur dioxide†
Abstract
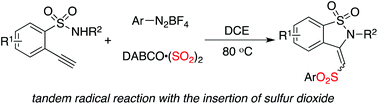
An efficient route to diverse 3-sulfonated-2,3-dihydrobenzo[d]isothiazole 1,1-dioxides is achieved through a three-component reaction of 2-ethynylbenzenesulfonamides, DABCO-bis(sulfur dioxide), and aryldiazonium tetrafluoroborates. The corresponding sulfonated benzosultams are produced in moderate to good yields. During the reaction process, the in situ generated arylsulfonyl radical via addition of an aryl radical to sulfur dioxide and the subsequent single electron transfer would be the key steps for the final outcome. DABCO acts as the carrier for single electron transfer, as well as a base to promote the C–N bond formation.

 Please wait while we load your content...
Please wait while we load your content...