Mechanistic investigation of Rh(i)-catalyzed alkyne–isatin decarbonylative coupling†
Abstract
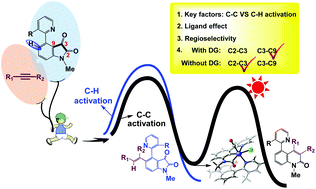
Rh-Catalyzed alkyne–isatin decarbonylative coupling provides an effective method for cleavage of C–C bonds in unstrained five-membered ring compounds. The challenge in this transformation is activation of the less-strained C–C bond, while avoiding competitive C–H activation. We performed DFT calculations to clarify this process to facilitate expansion of this strategy. The calculations show that chemoselectivity switching (C–C versus C–H functionalization) depends on the substituent on the phenyl ring of isatin. The coordination properties of the ligand significantly affect the alkyne insertion step. Dissociation of a strong σ-donor phosphine ligand from the Rh center to enable alkyne coordination is unfavorable, therefore the subsequent alkyne insertion step has a high energy barrier. Our calculations also explain the experimentally observed regioselectivity, which mainly arises from the interaction energy.


 Please wait while we load your content...
Please wait while we load your content...