Synthesis of 1,4-enyne-3-ones via palladium-catalyzed sequential decarboxylation and carbonylation of allyl alkynoates†
Abstract
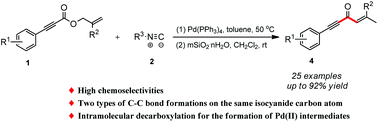
Herein we describe a novel protocol for the rapid assembly of 1,4-enyne-3-ones from isocyanides and 1,6-enyne. During this process, two different types of C–C bonds were formed on the same isocyanide carbon atom via sequential decarboxylation, insertion of isocyanide, and reductive elimination. Moreover, this approach shows advantages of mild reaction conditions and excellent functional group compatibility.


 Please wait while we load your content...
Please wait while we load your content...