Tuning liquid crystalline phase behaviour in columnar crown ethers by sulfur substituents†
Abstract
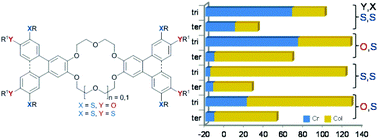
Novel [15]crown-5 and [18]crown-6 o-terphenyls differing in number and position of sulfur in the side chains and their corresponding triphenylene analogues were synthesized via a nucleophilic aromatic displacement of fluoride as the key step. Except for one short chain derivative all other compounds showed enantiotropic columnar mesophases which were studied by DSC, POM and XRD (WAXS, SAXS). The presence of sulfur induced broad room temperature columnar mesophases. This effect was more pronounced for bend [15]crown-5 derivatives than for linear [18]crown-6 derivatives. Redox properties were mostly governed by the triphenylene moieties, showing stepwise oxidation of the individual triphenylene units.
- This article is part of the themed collection: Novel π-electron molecular scaffolds


 Please wait while we load your content...
Please wait while we load your content...