Synthesis of 3-((arylsulfonyl)methyl)indolin-2-ones via insertion of sulfur dioxide using anilines as the aryl source†
Abstract
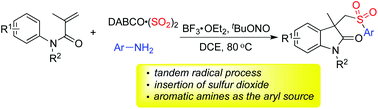
Facile assembly of 3-((arylsulfonyl)methyl)indolin-2-ones via insertion of sulfur dioxide starting from anilines, N-arylacrylamides and DABCO·(SO2)2 is developed. This one-pot reaction proceeds efficiently in dichloroethane, leading to sulfonated oxindoles in good yields. Anilines serve as the aryl source, and the in situ generated sulfonyl radicals from sulfur dioxide as the key intermediates are trapped by N-arylacrylamides to provide the corresponding products. Additionally, a broad reaction scope is observed under mild conditions.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...