Computational insights into the mechanisms of Au(i)-catalysed intramolecular addition of the hydroxylamine group onto alkynes†
Abstract
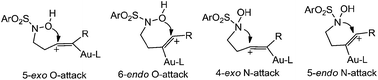
Computational studies were carried out to understand the reaction mechanisms and the origin of the substrate-dependent chemo- and regio-selectivities of the Au(I)-catalysed intramolecular addition of the hydroxylamine group onto alkynes. For the terminal and the phenyl-substituted alkynes, the 5-exo O-attack and the 5-endo N-attack have been proposed and rationalized to be the most favorable pathway, respectively, in the initial cyclization step. In the case of terminal alkyne substrates, the proposed α-oxo gold carbenoid intermediate might not be a key intermediate in the subsequent formation of 3-pyrrolidinone.

 Please wait while we load your content...
Please wait while we load your content...