Chemoselective acyl C–O bond activation in esters for Suzuki–Miyaura coupling
Abstract
Exploitation of the low binding affinity of NHC–palladium complex for the carbonyl oxygen of ester to form three-centered transition state led to the discovery of Suzuki–Miyaura coupling via unprecedented C(acyl)–O bond cleavage. The use of bulky NHC ligand resulted in direct coupling as it prevented decarbonylation of ester. This access to alternative chemoselectivity by careful selection has extended the synthetic portfolio of esters for the diversification of interesting molecular scaffolds.
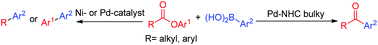
- This article is part of the themed collection: 2017 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...