Enantioselective synthesis of naturally occurring isoquinoline alkaloids: (S)-(−)-trolline and (R)-(+)-oleracein E†
Abstract
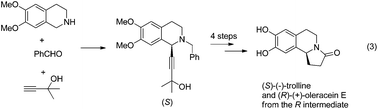
The highly efficient asymmetric syntheses of a pair of enantiomers, (S)-(−)-trolline and (R)-(+)-oleracein E, from commercially available 2-methyl-3-butyn-2-ol, benzaldehyde and 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline have been achieved in five steps with the catalytic enantioselective C1-alkynylation of 6,7-dimethoxy-3,4-dihydroisoquinoline as a key step, providing an efficient general approach for this type of naturally occurring chiral isoquinoline alkaloids.
- This article is part of the themed collections: Celebrating 70 Years of Shanghai Institute of Organic Chemistry and Organic Chemistry Frontiers HOT articles for 2017

 Please wait while we load your content...
Please wait while we load your content...