Iridium-catalyzed asymmetric hydrogenation of cyclic iminium salts†
Abstract
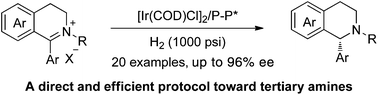
An enantioselective hydrogenation of cyclic iminium salts has been successfully realized by employing [Ir(COD)Cl]2 and chiral diphosphine ligands as catalyst, furnishing chiral N-alkyl tetrahydroisoquinolines and N-alkyl tetrahydro-β-carbolines with up to 96% ee and 88% ee, respectively. The hydrogenation provides a direct, simple and efficient protocol toward chiral tertiary amines. Meanwhile, asymmetric hydrogenation at the gram scale was also conducted smoothly without loss of reactivity and enantioselectivity.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...